Walking the Walk Selective Breeding for Mite Resistance; 2022 Update, Part 1
Contents
Resistance vs. Tolerance or “Survival” 1
Managed apiaries vs. natural evolution. 2
The necessity of Mechanical Agitators 3
So what’s our progress so far?. 10
A built-in lag inherent in open mating programs 13
Exhibit A: Mite-count tracking for our 2022 breeders 14
Walking the Walk
Selective Breeding for Mite Resistance; 2022 Update, Part 1
First published in ABJ June 2022
Randy Oliver
ScientificBeekeeping.com
I’ve been selectively breeding queens for many years. Breeding for color, gentleness, or AFB resistance was easy. Ditto for tracheal mite resistance, as well as for the ability to deal with Nosema ceranae. But breeding for varroa resistance has proven to be more difficult.
The obvious long-term solution to The Varroa Problem is to stock our hives with varroa-resistant bees. Varroa’s original host, Apis cerana, fits that bill. And left to their own, the hard hand of natural selective pressure applied to unmanaged bee populations has resulted in the evolution of resistant bees in various areas. But progress has been slower for those engaged in the selective breeding of managed European honey bees; their worldwide efforts are well reviewed by Le Conte [[i]]. So the question is, why is it taking so long for reliably mite-resistant bees to hit the market?
Resistance vs. Tolerance or “Survival”
Before I get going, please first allow me to clear up some terminology. I often hear people use the terms “resistance” and “tolerance” interchangeably. They actually have two very different biological definitions, as explained by Ayres and Schneider [[ii]]:
A host can evolve two types of defence mechanisms to increase its fitness when challenged with a pathogen — resistance and tolerance … Resistance is defined as the ability to limit pathogen burden while tolerance is defined as the ability to limit the health impact caused by a given pathogen burden. The sum of these two mechanisms defines a host’s defensive capacity.
There are “varroa tolerant” or “survivor” colonies, that may thwart the mite by maintaining tiny colony sizes, or by swarming or absconding frequently, or by fiercely attacking any invaders, but beekeepers are generally not interested in bees with those traits. Or “tolerant” bees may exhibit resistance to the mite-vectored viruses, even at high mite infestation rates.
But what I want are bees that take me back to the (pre-varroa) ”Good Old Days.” So I’m interested in bloodlines that “just say no” to varroa, and are by some means able to prevent the mites from successfully reproducing in their colonies.
Practical application: I’m breeding for resistance, not tolerance. A varroa-tolerant colony, due to its inability to control its mite population, will eventually suffer from the burden of its parasites and pathogens.
Managed apiaries vs. natural evolution
We might expect the hand of evolutionary pressure (natural selection) to come up with a “benign” mite, or a “stable” host-parasite relationship, but that’s not gonna happen so long as honey bees exist as managed livestock. This is because there’s no downside to varroa (or its associated viruses) if they inadvertently kill their host colony — so long as we keep providing fresh colonies to replace them.
In addition, our keeping of hives of bees closer than a mile apart means that mites can use the bees themselves as vectors to transmit those parasites to other hives in the vicinity. As pointed out by Ewald [[iii]], parasites that spread via flying vectors can be transmitted effectively from sick or dying hosts to new hosts, and therefore are not constrained from evolving to become even more virulent. (This would be the case whether we’re talking about the vector being mosquitoes, or drifting or robbing bees.) On the other hand, parasites that require their hosts to remain alive and healthy enough to transmit the parasite vertically to their offspring (in the case of bees, to their swarms), may develop a stable host-parasite relationship (as in the case of humans and our body lice or follicle mites).
Practical application: The above means that there’s no evolutionary pressure upon either varroa or Deformed Wing Virus to become less virulent — The Varroa Problem is not going to get better until we start keeping resistant bees. Thus our only long-term solution is to select for honey bee stocks that are able to reduce varroa’s reproductive success to the extent that it becomes only a minor pest in the colony. Selective breeding is simply human-directed evolution; in this case working with Nature for the benefit of our beloved (and beleaguered) honey bees.
Background
For those who have not been following my “Walking the Walk” series on breeding for varroa resistant bees, you can catch up here [[iv]].
Practical application: My sons and I are running a long-term proof of concept experiment, for the benefit of the commercial queen breeders/producers. My goal is to determine the plausibility/feasibility, labor cost, and rate of progress from a simple “traditional” breeding program, starting with one’s own mongrel stock.
Our experimental program involves managing a dedicated breeding population, by the open-mating (within that breeding population) of a yearly cohort of at least 1000 new queens, all bred from roughly 30 selected breeder queens from the previous year. The selected breeders each spring are chosen from colonies that exhibited the ability to hold their mite infestation rate to near zero over the course of an entire year (without any treatments), but just as importantly, continue to have the desirable properties of being gentle and productive.
I’m intentionally avoiding instrumental insemination, genetic analysis, brood dissection, diligent maternal line tracking, or other costly or time-consuming methods. Our only assessment method is taking mite washes (roughly 2000 per year), coupled with visual inspection for general colony qualities. Any colonies rejected as potential breeders are treated; no colonies need be lost to varroa.
The necessity of Mechanical Agitators
The key to this method is to be able to perform rapid-fire mite washes. This requires portable mechanical agitators, and a system that allows a crew to work together without confusion, described at [[v]]. Since that article, I’ve spent many hundreds (my wife says thousands) of hours in the shop, perfecting new generations of portable mite wash agitators (Figure 1).

Fig. 1 I built several of these Generation 2 portable mite wash agitators. My assistant Brooke Molina has her finger over the start button. The agitator then performs ~300 revolutions and shuts itself off after 60 seconds. We’ve validated that this agitation recovers of at least 95% of the mites.
The agitators above have served us very well, but when we shifted from using alcohol to Dawn detergent instead, I realized that I could improve them further. After building, testing, and fine-tuning many designs and prototypes, I’ve now developed Generation 4, with better cups, and a simpler design (Figure 2).

Fig. 2 The Gen 4 agitator is specifically designed for use with Dawn detergent, as well as for beekeepers to be able to build their own from off-the-shelf components. This model utilizes a sturdier screw-top jar, and we’ve validated 99% mite recovery in 60 seconds. It includes a pull-out magnifying mirror and holder for rapid counting of mites. I intend to publish the plans in an upcoming article, and may even make up kits for assembly.
Figure 2 shows the agitator sitting on a pull-out table that I built in the back of my Honda CRV, set up for us to rapidly get going when we pull into a yard. With these portable agitators, we can perform mite washes (including opening the hive, taking the sample, closing the hive, agitation and counting, and writing the mite count on top of the hive) at the rate of 3-4 man-minutes per hive (a crew of three of us can easily determine the mite count for every colony in a yard of 48 hives in less than an hour). At $25 per hour, that works out to about a buck and a half labor cost to determine each colony’s infestation rate (that’s less than the cost of some treatments). And we can make an immediate management decision for each colony.
Practical application: Without such portable agitators, performing the number of mite washes necessary for our breeding program wouldn’t be feasible. We find that our savings from not spending money on unneeded mite treatments, as well as the identification of colonies that need more than “the usual” treatment (or that have other issues), more than pays for the cost of labor involved in performing the mite washes.
The resistant colonies
The thing that keeps us going with this demo experiment is the beauty of the colonies that we’ve identified as being mite-resistant — which, if we hadn’t performed mite washes on them, we wouldn’t have known even existed in our operation! (Figure 3).

Fig. 3 The index card on this booming double-deep colony after almonds (with an undisturbed green drone frame), shows the counts for mites in samples of a half cup of bees, taken over the course of a year. (We allowed the mites to build up from the starting nuc in April until the first wash in July.) It’s not unusual for the count to spike in November as the colonies briefly go broodless. Even the count of 7 is only about a 2% infestation rate as the bees go into winter. In the case of this colony, the count expectedly dropped again by the March assessment, after the colony had returned from pollination, at which time most of the mites were back in the brood. For what it’s worth, although it needed no treatments, this colony would not have passed the bar to make it as a breeder.
Of our “potential breeder” pool of resistant colonies each year, to stay in the running, a colony must not only maintain a mite count of near zero for the course of an entire year, but also be gentle, healthy, build up quickly, and be productive as far as honey. That subsequently eliminates a goodly portion of those colonies marked as “potential breeders” at the first assessment.
We take most all of our potential breeders to almond pollination. When I take their mite counts in March, any that are not busting at the seams with bees, or that did not put on plenty of honey in the orchards, are no longer considered as breeders.
Practical application: The remaining breeder pool that we actually graft from (around 30) are the queens of colonies that not only completely controlled varroa, but that any beekeeper would be delighted to have in their own operation, as evidenced by the responses of the several large professional beekeepers who have assisted me in breeder selection (Figure 4).

Fig. 4 This colony laughed at varroa. Brooke said it all one day when she quipped “Zeroes are heroes.” Note the lack of a November spike in count. We didn’t recover a single mite in any of the four samples taken from this colony. If its count was still 1 or less in March, it would be considered as a breeder (at low mite infestation rates, there’s virtually no statistically-significant difference between a count of 1 or 0).
Practical application: The mite counts of this colony are impressive, since a non-resistant untreated colony under our management system will typically reach a mite count of well over 50 by September. Unfortunately, we’ve found that the mite resistance of a colony as a whole does not necessarily predict the performance of daughter colonies. For most of these multiple-zero colonies, daughters bred from their queens only exhibit the breeding population average for resistance. So far we haven’t seen a strong degree of heritability for the trait.
The above frustrating fact may be about to change, since the daughters of a couple of last year’s queens exhibited strong heritability, with up to 50% of their daughter colonies showing resistance. You may have guessed that we’ll be breeding heavily from them this season! In any case, we’re continually impressed by how well our Zero Heroes perform.
PROOF OF CONCEPT: Our mite-resistant colonies are often the strongest and most productive colonies in a yard. Many ask me whether we sacrifice gentleness for mite resistance (Figure 5).

Fig. 5 Eric snapped a shot of me as I performed August mite washes in hot weather. I often knock out 50 mite washes in an afternoon. It’s easy to perform the bioassay for gentleness — if I start getting stung, that colony doesn’t make grade.
So what’s our progress so far?
In a recent project by De la Mora [[i]], working with the Ontario Queen Breeders Association, they appeared to obtain a substantial increase in mite resistance after only two generations, using a selection and breeding method similar to mine. That rate of success is better than that of any other breeding program that I’m aware of, so I hope that their good luck continues! For the rest of us, it’s a much slower slog. My sons and I are going into our sixth year of very strong selection.
I recently realized that we may have been inadvertently kicking a proportion of potentially-resistant colonies out of the program at our first mite wash assessment in June or July, when we go ahead and treat any colony with a count of more than 1 mite (we rejected anything above zero in our first years). At that low of an infestation rate (1 mite per 315 bees equals a 0.317% infestation rate — that’s less than a third of a percent), we can calculate the probability of getting different mite wash counts (Figure 6).
[i] De la Mora A, et al. (2020) Selective breeding for low and high Varroa destructor growth in honey bee (Apis mellifera) colonies: Initial results of two generations. Insects 11(12):864.
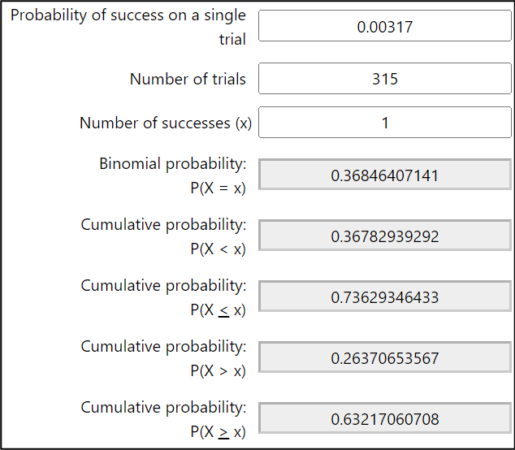
Fig. 6 I used the binomial calculator at stattrek.com to work the figures. At an average mite infestation rate of a 1 mite per 315 bees (probability of 0.00317), the chances of getting a wash count of zero is 37%; of seeing exactly 1 mite also 37%; leaving a 26% chance of getting 2 or more mites in a wash. That’s a 26% chance of rejecting a potential breeder right off the bat!
Since our records indicate that roughly half the colonies with mite counts of 1 or 0 in June maintain counts below 7 by the next spring, that means that we’ve likely been excluding (and treating) a lot of what were actually resistant colonies, since we’ve been rejecting a very large number of colonies in June or July that had counts of 2 (Figure 7).

Fig. 7 Out of curiosity, I didn’t treat a few colonies that had mite counts of 2 in early July last year to see where they’d go. The above tag answered that question for this colony! My guess is that we’ve been overlooking a large number of colonies that were actually resistant.
Practical application: It’s a goodly amount of work to perform the first washes on well over 1000 hives in June or July. We’ve applied severe and unsparing selective pressure at the first wash, in order to reduce the number of hives to follow up on. But as with Dr. John Kefuss’ experience, we’ve also observed that resistant colonies are often able to take mite counts back down on their own. So it boils down to how many potential breeders you want to track with additional washes. Luckily, even those resistant but rejected (and thus treated) colonies will still wind up producing drones for next year’s matings.
So where do we stand? Last year was a tough year for our bees. They got hit by severe drought, forest fires, flooding, snow and falling tree damage, bear damage, and then a cold snowstorm just after they started ramping up broodrearing prior to almond pollination. We lose very few colonies to mites, but last year the environment was devastating to our bees. Eric and Ian were barely able to fill our pollination contracts for almonds (although those that went graded well).
That said, of the thousand-plus colonies returning from almonds, we still had 126 tracked (and untreated) potential breeders that exhibited mite wash counts in March of less than 7 (less a 2% infestation rate after an entire year without treatment). Figuring in weather, fire, and bear losses of marked potential breeders, and factoring in our over-culling at our first summer wash, I estimate that between 15% to 20% of our 2021 colonies exhibited strong mite resistance (Figure 8).
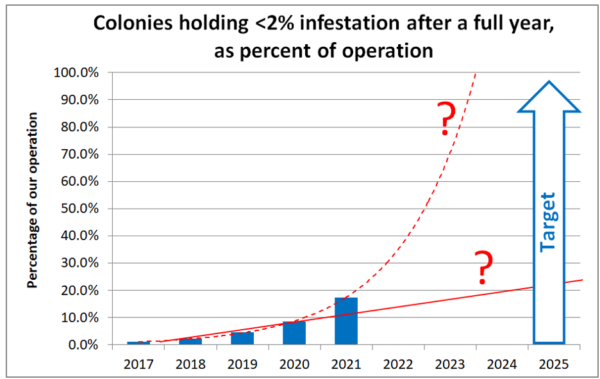
Fig. 8 The above chart reflects my “best guess” proportions of varroa-resistant colonies in our operation over the years. And in one yard this year, we hit 50%. We’ve still got a ways to go until we get to the 95% level at which I might start claiming that our stock is “mite resistant,” but our progress to date is encouraging. Will we slowly make linear progress (the solid red line), or will our results continue to track exponentially (the dashed red line)? I’ll return to this question in my next article.
Practical application: Compared to selective breeding programs to improve the productivity of agricultural crops or animals, which involve changing the morphology, physiology, and energy efficiency of the organisms, to breed for mite resistance, we may only need to flip a couple of behavioral switches. Maybe someone snuck some cannabis leaf into my smoker, but I’m crossing my fingers that our progress will continue to track the dashed line!
A built-in lag inherent in open mating programs
In a breeding program, one can perform either positive selection or negative selection (or a combination of the two). In our own breeding population, in which 99%+ of the colonies would be considered as being “gentle,” it’s easy to practice negative selection against the occasional queen whose colony turns out to be a bit spicy. On the other hand, if we were breeding solely for honey production, we might positively select as breeders only the queens from the few colonies that put on exceptional amounts of honey. In the case of breeding for varroa resistance, we’re doing both — breeding only from the queens whose colonies were exceptionally good at keeping mites in check (positive selection), while simultaneously removing from the breeding population any queens whose colonies appeared to require treatment or didn’t make much honey (negative selection). But unfortunately, there’s a built-in two-year lag for such negative selection, which acts as a drag against the rate of progress of our program.
Understand that the genetics of drones come only from their mothers (whose alleles in turn consist of a mixture of those from their own mothers and fathers). Therefore, since we breed only a single generation of queens each year, the alleles of the drone pool that they will mate with will come from those drones’ grandfathers and grandmothers — reflecting the genetics of the breeding population from two years earlier. Thus our “directed evolution” of the genetics of the drone pool will always lag behind that of the genetics of the breeder queens selected each year. The genetics of the females of the resulting colonies come 50% from the chosen breeder queens of that year, and 50% from the drones whose genetics reflect that of their grandparents. (Yes, go ahead and reread and digest this paragraph.)
Practical application: In a natural breeding population, this genetic conservation of alleles from several generations back helps to prevent dangerous genetic bottlenecking in the case of a sharp reduction in the size of a breeding population of bees due to environmental events such as severe drought or other weather event, landscape-scale fire, or a devastating disease (the stored mixture of spermatozoa in any surviving queen’s spermatheca conserves the local population’s genetic diversity). Unfortunately, this built-in conservation of alleles also slows down the progress of any human-directed selective breeding program that uses open mating, since the alleles of each year’s drone pool reflects the alleles of the population from two years earlier.
Exhibit A: Mite-count tracking for our 2022 breeders
So after taking mite washes after almonds, I needed to select this year’s breeders. I’m guessing that you might like to see how their mite counts tracked over the course of the year (Figure 9).
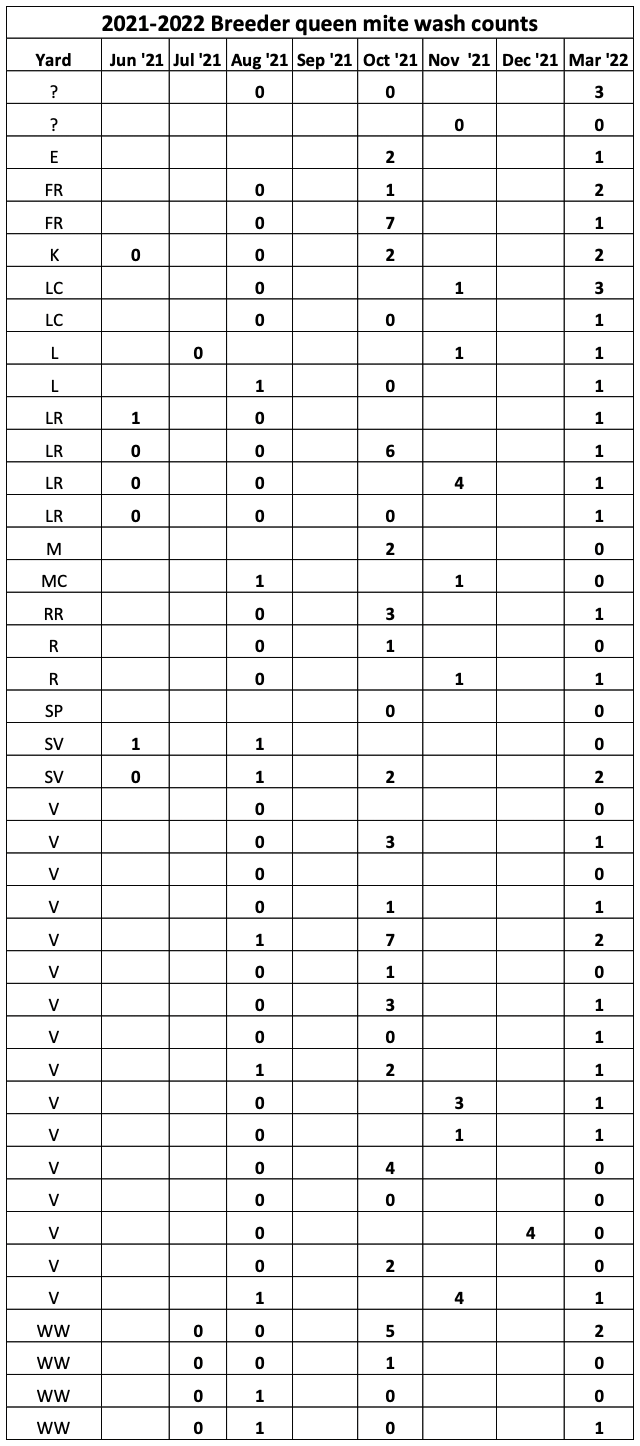
Fig. 9 In 2021, in many of our yards mite counts didn’t get high enough for selection until July or even August, and some didn’t even get checked until November. Note that over a third of this season’s breeders exhibited mite counts of zero after a full year without treatment (their prior count histories indicate that those zero counts in March weren’t flukes).
The yard abbreviations indicate different queen bloodlines, so in order to maintain genetic diversity, I included breeders from a diversity of yards, which required me to include a few with March counts of above 1. We will again requeen our entire operation this year with daughters from these queens, especially those from Yard V (in which 24 of the 48 colonies exhibited strong resistance). I’m especially interested in that bloodline, which I plan to use for some isolated-yard inbreeding later this season.
Coming
Next month I’ll take a dive into our resistant colonies’ mechanism for resistance and the genetics involved.
References
[1] Le Cont, Y, et al. (2020) Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 11: 873. doi:10.3390/insects11120873
[2] Ayres, J & D Schneider (2008) Two ways to survive an infection: what resistance and tolerance can teach us about treatments for infectious diseases. Nat Rev Immunol. 8(11): 889–895.
[3] Ewald, P (2004) Evolution of virulence. Infectious Disease Clinics 18(1): 1-15.
[4] 2017 plan: https://scientificbeekeeping.com/the-varroa-problem-part-6a/
2018 update: https://scientificbeekeeping.com/selective-breeding-for-mite-resistance-1000-hives-100-hours/
2019 update: https://scientificbeekeeping.com/selective-breeding-for-mite-resistance-walking-the-walk/
[5] The Varroa Problem: Part 10-Smokin’-Hot Mite Washin’ – Scientific Beekeeping
[6] De la Mora A, et al. (2020) Selective breeding for low and high Varroa destructor growth in honey bee (Apis mellifera) colonies: Initial results of two generations. Insects 11(12):864.



